Tel:+86 180 6702 9687 / +86 136 1159 5862E-mail:wuth@pskbio.com / fengmeng@pskbio.com

Nucleic acid therapeutics have reached a critical inflection point in the treatment of central nervous system (CNS) diseases. Across neurodegenerative and rare neurological indications—including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS)—antisense oligonucleotides (ASOs) and siRNA programs have repeatedly demonstrated robust target engagement and biomarker modulation in clinical studies.
These results confirm a key industry consensus: the biological targets are valid, but delivery remains the limiting factor. Most CNS RNA drugs still rely on intrathecal administration, an invasive and resource-intensive approach that constrains patient compliance, treatment duration, and ultimately commercial scalability. Meanwhile, delivery technologies that have succeeded in peripheral tissues, such as lipid nanoparticles (LNPs), face fundamental challenges in the CNS, including limited brain exposure, inflammatory risk, and insufficient intracellular delivery efficiency.
As a result, the future growth of CNS RNA therapeutics will be defined not by target discovery, but by the emergence of delivery platforms capable of enabling safe, repeatable, and systemic brain delivery.
Exosomes are naturally occurring nanoscale vesicles with intrinsic advantages as CNS delivery vehicles. Compared with synthetic or inorganic nanoparticles, exosomes offer:
· Low toxicity and low immunogenicity
· High biocompatibility and biodegradability, without long-term tissue accumulation
· Natural ability to cross biological barriers, including the BBB
· Compatibility with repeated and long-term dosing, a key requirement for chronic CNS diseases
· Engineering flexibility at both the surface and cargo levels, enabling enhanced brain targeting and efficient loading of multiple RNA modalities, including siRNA, miRNA, ASOs, and mRNA
With growing industrial validation and multiple programs advancing toward IND-enabling and early clinical development, exosomes represent a versatile, scalable delivery platform with strong translational precedent and significant long-term potential in CNS therapeutics.
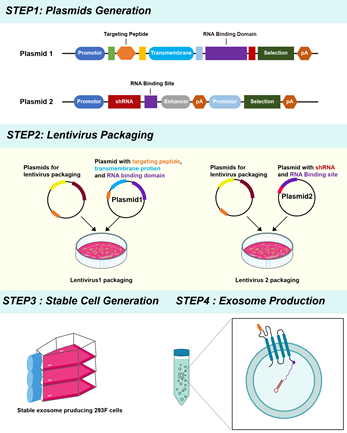
P.S.K Biosciences has developed a proprietary engineered exosome platform designed specifically to overcome CNS delivery barriers.
Dual technological barriers form the core of the platform:
1. Brain-targeting exosome surface engineering – Through genetic engineering, brain-targeting peptides are displayed on the exosome membrane, enabling preferential accumulation in the CNS following peripheral administration.
2. Optimized nucleic acid loading and stability – By co-optimizing exosome-associated loading proteins and nucleic acid sequence design, P.S.K Biosciences significantly improves siRNA loading efficiency, stability, and functional delivery.
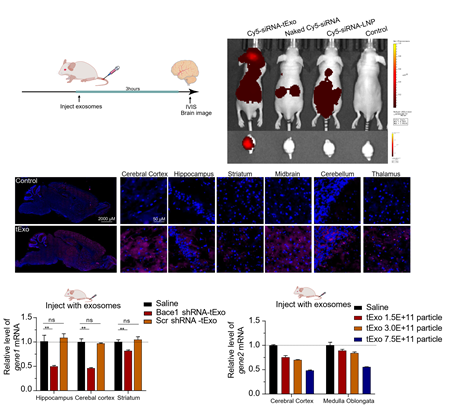
Breakthrough in vivo performance:
· Supports systemic administration, including intravenous injection, fundamentally avoiding the limitations of intrathecal dosing
· Achieves a brain-to-blood ratio of 2.0, approximately 10-fold higher than conventional delivery approaches
· Demonstrates robust and reproducible target mRNA knockdown in the brain in animal models, confirming functional intracellular delivery
Engineered exosomes do not change the mechanism of action of nucleic acid therapeutics; instead, they represent a critical delivery upgrade that enables existing and future RNA drugs to reach their full potential.
· Administration innovation: Peripheral dosing routes such as intravenous injection improve patient compliance and long-term treatment feasibility.
· Commercial scalability: Systemic delivery supports broader patient populations and chronic indications, significantly expanding market potential.
The development of CNS nucleic acid therapeutics can be viewed in two stages: first, target validation—successfully achieved by ASOs and siRNAs; second, delivery innovation. P.S.K Biosciences is positioned at the forefront of this second stage.
As challenges in safety, large-scale manufacturing, and quality control continue to be addressed, P.S.K Biosciences’s engineered exosome platform has the potential to enable more accessible, durable, and scalable therapies for patients with devastating neurological diseases.
Link to the Original Article: